A study of potassium acetate concentration on clay structure
DOI:
https://doi.org/10.60136/bas.v4.2015.295Keywords:
Intercalation, Potassium acetate, Kaolin expansionAbstract
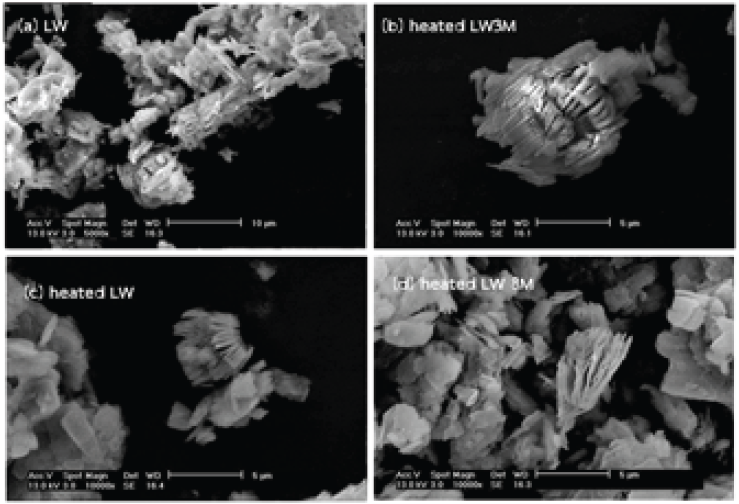
This research study aims to study the potassium acetate concentrations on the increase of kaolin interlayers through intercalation and heat treatment processes. The effect of six different concentrations of potassium acetate : KAC (3, 4, 5, 6, 7, and 8 M) were used. The soaking process was applied on clay to form clay-acetate complex. X-ray diffraction (XRD) results indicated that the higher Kac concentration caused the higher intercalation ratio. However, a concentration of 5 M KAC was chosen to gain an optimum intercalation ratio if green chemistry was taken into account. The clay-intercalating complex was carried out completely at room temperature for 3 days. The complex was heat treated to delaminate the expanded layer. SEM and XRD confirmed that Kac especially influence on kaolin structure by expanding the kaolin layer.
References
MURRAY, H.H. Overview : clay mineral applications. Appl. Clay Sci., 1991, 5(5-6), 379-395.
CHENG, H., et al. The thermal behavior of kaolinite intercalation complex- A review. Thermochim. Acta., 2012, 545, 1-13
LAGALY, G., M. OGAWA, and I. DEKANY. Clay mineral organic interactions. Handbook of Clay Science. Elsevier. 2006
CHENG, H., et al. Influencing factors on kaolinite-potassium acetate intercalation complexes. Appl. Clay Sci., 2010, 50(4), 476-480.
CASTELLANO, M., et al. Bulk and surface properties of commercial kaolins. Appl. Clay Sci., 2010, 48, 446-454.
CHENG, H., et al. Mechanism of dehydroxylation temperature decrease and high temperature phase transition of coal bearing strata kaolinite intercalated by potassium acetate. J. Colloid Interface Sci., 2012, 376(1), 47–56.
YU, X., et al. The intercalation of cetyltrimethylammonium cations into muscovite by a two-step process: II. The intercalation of cetyltrimethylammonium cations into Li-muscovite. J. Solid State Chem., 2006, 179(5), 1525–1535.
CHENG, H., et al. Delamination of kaolinite-potassium acetate intercalates by ball-milling. J. Colloid Interface Sci., 2010, 348(2), 355-359.
ELBOKL, T.A., and C. DETELLIER. Intercalation of cyclic imides in kaolinite. J. Colloid Interface Sci., 2008, 323, 338-348.
LETAIEF, S., et al. Nanohybrid materials from interlayer functionalization of kaolinite. Application to the electrochemical preconcentration of cyanide. Appl. Clay Sci., 2008, 42(1-2), 95–101.
MAKÖ, É., G. RUTKAI, and T. KRISTÖF. Simulation-assisted evidence for the existence of two stable. kaolinite/potassium acetate intercalate complexes. J. Colloid Interface Sci., 2010, 349(1), 442–445
อดุลย์ ทรายตัน, การใช้กากดินขาวลําปางเป็นส่วนผสมในการผลิตผลิตภัณฑ์เครื่องปั้นดินเผาชุมชนบ้านม่อนเขาแก้ว จังหวัดลําปาง รายงานการวิจัย, มหาวิทยาลัยราชภัฏลําปาง, 2554
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Bulletin of Applied Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.









