Browning kinetics of egg white during high-temperature process
DOI:
https://doi.org/10.60136/bas.v12.2023.647Keywords:
Egg white product, Browning reaction, Glucose oxidase, Kinetic modelAbstract
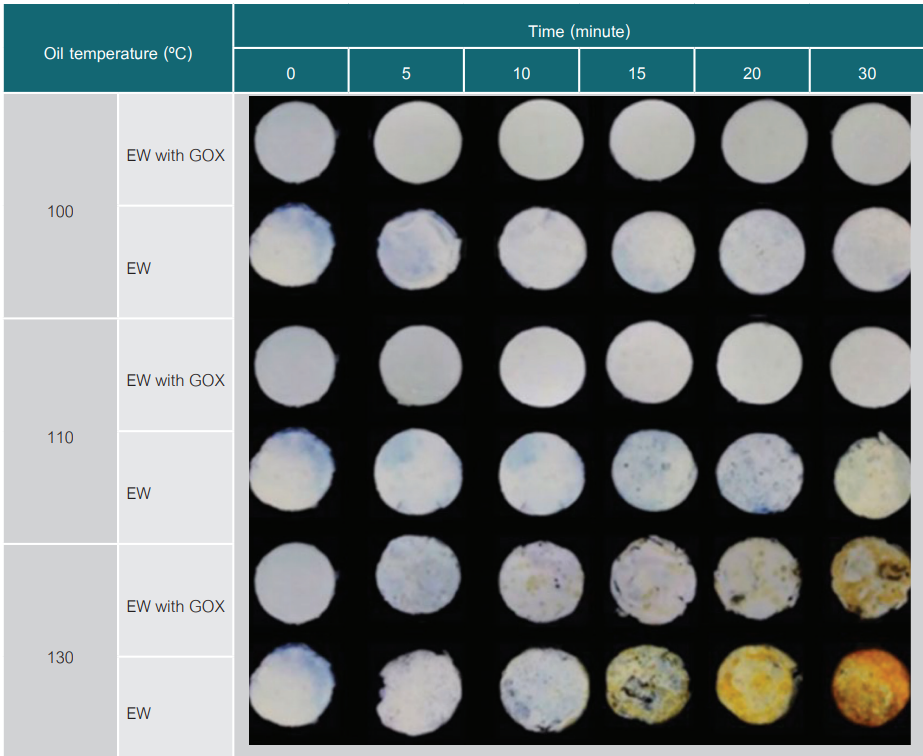
Egg white product is commonly produced by pasteurization to avoid brown color development and needs to be kept in a refrigerator. To explore the opportunity of producing shelf-stable egg white products, this research aimed to develop the browning kinetic model of egg white undergoing a high-temperature treatment and to minimize the browning reaction by desugaring with glucose oxidase. Egg white (8 mL) was filled in sample holders equipped with a thermocouple. The sample holders were preheated in a water bath at 70°C for 30 minutes to solidify the sample and then heated in an oil bath at 100, 110, and 130°C for 30 minutes. The color of the sample surface was measured using a spectrophotometer. The yellowness index (YI) was calculated to identify the degree of brown color development. It was found that the L* value of egg white declined, while a* and b* values increased with increasing heating time. The first-order kinetic model was applicable for predicting the change of YI-ratio (R2 = 092-0.95, RMSE = 0.11-029). In addition, the samples pre-treated with glucose oxidase of 30 units/mL-egg white and 30°C-incubation for 6.5 hours had a significant reduction of the YI-ratio from 5.9 to 2.6. This research indicates the potential for egg-white production at a high temperature with lighter color by adding glucose oxidase.
References
Lechpattanaporn K, Wang Y, Wang J, Tang J, Hallberg LM, Dunne CP. Sterilization of scrambled eggs in military polymeric trays by radio frequency energy. J Food Sci. 2005;70(4):E288–E294.
Pastoriza S, Quesada J, Rufián-Henares, JA. Lactose and oligosaccharides: Maillard reaction. Reference Module in Food Science [Internet]. 2018 [cited 2023 Jan 5];1-8. Available from: https://doi.org/10.1016/B978-0-08-100596-5.22552-3
Zhang W, Tang J, Liu F, Bohnet S, Tang Z. Chemical marker M2 (4-hydroxy-5-methyl-3 (2H)-furanone) formation in egg white gel model for heating pattern determination of microwave-assisted pasteurization processing. J Food Eng. 2014;125:69-76.
Bornhorst ER, Tang J, Sablani SS, Barbosa-Cánova GV. Development of model food systems for thermal pasteurization applications based on Maillard reaction products. LWT - Food Sci Technol. 2017;75:417-24.
Llave Y, Takemori K, Fukuoka M, Takemori T, Tomita H, Sakai N. Analysis of browning of broiled foods by noncontact techniques: A case study for Japanese eggplant (Solanum melongena). J Food Process Eng. 2017;40(1):e12347.
Scott D. Food stabilization, glucose conversion in preparation of albumen solids by glucose oxidase-catalase system. J Agric Food Chem. 1953;1(11):727-30.
Quan TH, Benjakul S. Impacts of desugarization and drying methods on physicochemical and functional properties of duck albumen powder. Dry Technol. 2019;37(7):864-75.
Sisak C, Csanádi Z, Rónay E, Szajáni B. Elimination of glucose in egg white using immobilized glucose oxidase. Enzyme Microb Technol. 2006;39(5):1002-7.
Bali OD, Mittal GS. Kinetics of tofu color changes during deep-fat frying. LWT-Food Sci Technol. 2003;36(1):43-8.
Matsuda H, Llave Y, Fukuoka M, Sakai N. Color changes in fish during grilling–Influences of heat transfer and heating medium on browning color. J Food Eng. 2013;116(1):130-7.
Llave Y, Fukuda S, Fukuoka M, Shibata-Ishiwatari N, Sakai N. Analysis of color changes in chicken egg yolks and whites based on degree of thermal protein denaturation during ohmic heating and water bath treatment. J Food Eng. 2018;222:151-61.
ASTM International. Standard practice for calculating yellowness and whiteness indices from instrumentally measured color coordinates. Paint-tests for chemical, physical, and optical properties. ASTM E313-05. West Conshohocken, PA : ASTM International; 2005.
Dunn WC. Temperature and Heat. In: Introduction to instrumentation, sensors, and process control. Norwood, Massachusetts: Artech House Inc; 2006.p.149–155.
Boekel MAJS van. Kinetic aspects of the Maillard reaction: a critical review. Food/ Nahrung. 2001;45(3): 150–9.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Bulletin of Applied Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.









