The studies of hydrolytic resistance of inner surfaces of glass containers for energy drinks
DOI:
https://doi.org/10.60136/bas.v3.2014.182Keywords:
Chemical Resistance, Glass Container, Hydrolytic testAbstract
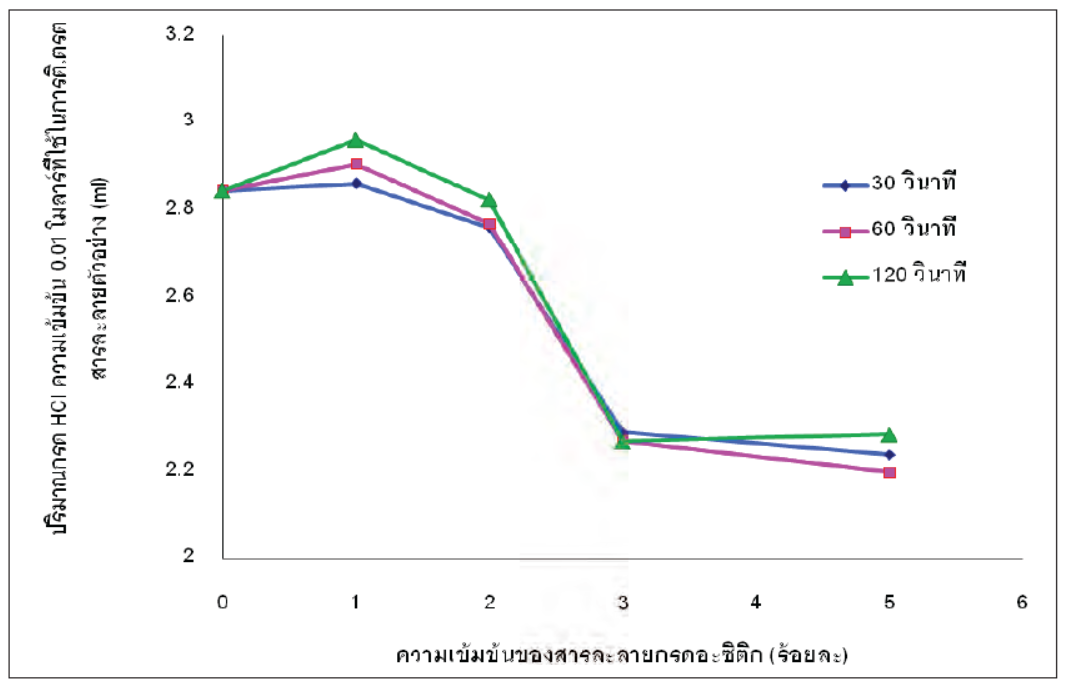
Nowadays, the market of the energy drinks is expanding quite substantially. From the safety point of view, the contamination of heavy elements and glass composition elements which might be dissolved by their solution is concerned. The glass with a high hydrolytic resistance property can decrease the contamination of those elements. The objective of this study was to improve the hydrolytic resistance of the energy drink glass containers by means of treating the inner surfaces with 1, 2, 3 and 5% of acetic acid. Hydrolytic tests were conducted in accordance with ISO 4802-1. It was found that the hydrolytic resistance increased after the acid treating. The treatment condition of 3% of acetic acid for 30 second resulted in the highest hydrolytic resistance. The treated and un-treated bottles were filled up with citric acid solution at pH 3.5 (close to the pH of energy drink), kept at room temperature (about 25-27 C) and 20°C for 7, 14, 21 and 28 days soaking time. The leached elements were analyzed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). The results showed that the amount of leached heavy metals, i.e. As Cd Cr Pb Hg Se Sb, and Ca k which were the compositions in glass, were not found. Only leached Na was found. The results also showed that the contents of leached sodium from the treated and un-treated bottles were not different at the same storage temperature. However, the amount of leached sodium element (Na) of the bottles kept at room temperature was higher than those kept at 20°C. The highest content of leached sodium was found at 21 days soaking time. In summary, the hydrolytic resistance of glass surfaces for the solution at pH 3.5 was decreased when the temperature and the soaking time were Increased. average firing shrinkage, the standard deviation and the change of the shrinkage with temperature.
References
SHELBY, J. E. Introduction to glass science and technology. 2nd ed., UK: The Royal Society of Chemistry, 2005
เทพีวรรณ จิตรวัชรโกมล. สาเหตุและการ ป้องกันสนิมแก้วในอุตสาหกรรมแก้ว. วิทยานิพนธ์ วท.ม. (เทคโนโลยีวัสดุ). กรุงเทพฯ : บัณฑิตวิทยาลัย จุฬาลงกรณ์มหาวิทยาลัย, ถ่ายสําเนา. (2539)
REIMANN, C., M. BIRKE and P. FILZMOSER. Bottled drinking water: water contamination from bottle materials (Glass, Hard PET, Soft PET), the Influence of colour and acidification (Online) (Viewed 12 July 2012). Available from: http://www.statistik.tuwien.ac.at/public/filz/papers/10APGEO.pdf.
ธีรศักดิ์ ตั้งกิจติมศักดิ์. การเพิ่มความทนทาน ต่อสารเคมีของบรรจุภัณฑ์แก้วสําหรับเครื่องดื่ม โครงงานวิศวกรรมวัสดุ, วศ.บ. (วิศวกรรมวัสด), กรุงเทพ: มหาวิทยาลัยเกษตรศาสตร์, 2552.
INTERNATIONAL ORGANIZATION FOR STANDARDIZATION. ISO 4802-1: 2010, Glassware-Hydrolytic resistance of the Interior surfaces of glass containers – Part 1: Determination by titration method and classification.
CONRADT, R. Chemical durability of glass (Online). (Viewed 8 April 2013). Available from: http://lib3.dss.go.th/fulltext/glass/WEB/web_ dr_kanit/CU_GLASS_COURSE_DURABIUTY.pdf
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Bulletin of Applied Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.









