Preparation of ceramic pigment from tanned leather waste
DOI:
https://doi.org/10.60136/bas.v11.2022.125Keywords:
Ceramic pigment, Tanned leather waste, Preparation of ceramic pigmentAbstract
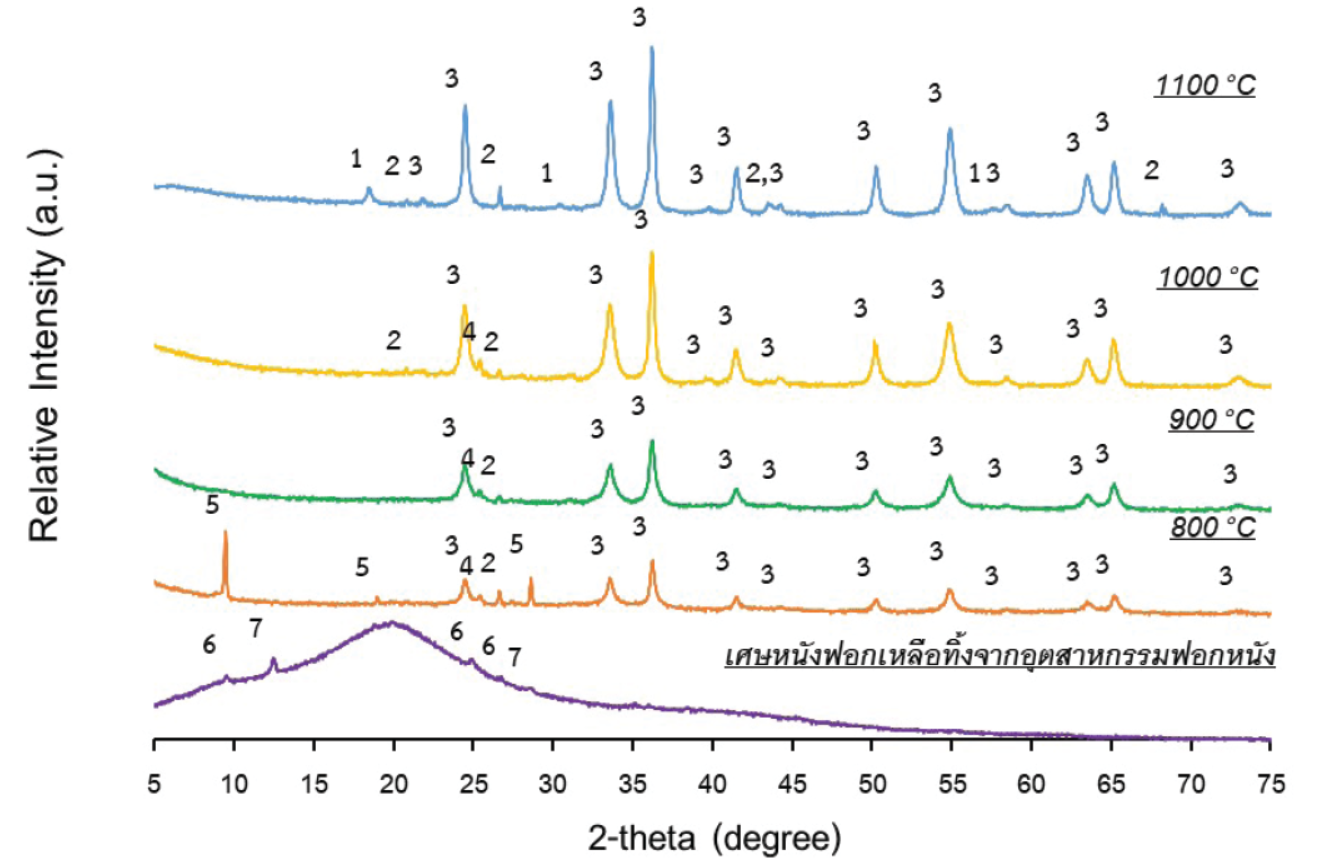
This research studies on the utilization of tanned leather waste from tannery industries composed of chromium for producing the ceramic pigment.Firstly, the chemical composition and phase composition of the waste were characterized using X-ray fluorescence (XRF)and X-raydiffraction (XRD), respectively. The waste fired at 1100 °C, which the main phase was chromium (II) oxide (Cr2O3), was used as chromium source to create the ceramic pigment. The fired waste was wet-mixed with ZnO and Al(OH)3 to create the pink pigment (Zn-Al-Cr system) and with ZnO and Fe2O3 to create the brown pigment (Zn-Fe-Cr system).The mixtures were then fired at 1200๐C before using as ceramic pigment in a glazed stoneware. 10 wt%of the fired waste and the prepared pigments were separately added into the transparent glaze and were then coated on the biscuit samples before firing at 1200 °C. The results shown that the glazed samples (green, pink, and brown) were successfully produced the ceramic pigment using the tanned leather waste as chromium source. Moreover, the leaching of Lead (Pb), Cadmium (Cb) and Chromium (Cr) from glazed product were detected.
References
NOGUEIRA, Francisco G.E., Isabela A. CASTRO., Ana R.R. BASTOS., Guilherme A.SOUZA., Janice G. De. CARVALHO., and Luiz C.A.OLIVEIRA. Recycling of solid waste rich in organic nitrogen from leather industry: Mineral nutrition of rice plants. Journal of Hazardous Materials. 2011, 186(2-3), 1064–1069.
BETHELHEM HAILE TESEMA. Tannery Solid Waste Generation Rate and Preparation of Leather Board From chrome Shaving Waste and Plant Fibers “A Wealth from Waste Approach for Leather Industry”. Degree of Master of Science, Environmental science Addis Ababa University. 2018.
SATHISH, M., B. MADHAN., and J.R. RAO. Leather solid waste: An eco-benign raw material for leather chemical preparation – A circular economy example. Waste Management. 2019, 87, 357–367.
คชินท์ สายอินทวงศ์. สีเซรามิก(Color stain) [ออนไลน์]. 2551. [อ้างถึงวันที่ 20 กุมภาพันธ์ 2565]. เข้าถึงจาก: http://www.thaiceramicsociety.com/rm_paint_ceramiccolor.php.
วรรณา ต.แสงจันทร์. เอกสารผลงานวิจัยเรื่องการวิจัยและพัฒนาสีเซรามิกชนิดสปิเนลสีน้ำตาล และสีชมพู. กรุงเทพฯ: กลุ่มวิจัยและพัฒนา ศูนย์วิจัยและพัฒนาอุตสาหกรรมเซรามิก กรมวิทยาศาสตร์บริการ, 2542.
OZEL, E., and S. TURAN. Production and characterization of iron-chromium pigments and their interactions with transparent glazes. Journal of the European Ceramic Society. 2003, 23(12), 2097–2104.
VERGER, L., O. DARGAUD., N. MENGUY., D. TROADEC., and L. CORMIER. Interaction between Cr-bearing pigments, and transparent glaze: A transmission electron microscopy study. Journal of Non-Crystalline Solids. 2017, 459, 184-191.
ABREU, M.A., and S.M. TOFFOLI. Characterization of a chromium-rich tannery waste and its potential use in ceramics, Ceramics International. 2009, 35(6), 2225–2234.
TAHIRI, S., A. ALBIZANE., A.MESSAOUDI., M. AZZI., J.BENNAZHA., S.A.YOUNSSI., and M.BOUHRIA. Thermal behaviour of chrome shavings and of sludges recovered after digestion of tanned solid wastes with calcium hydroxid. Waste Management. 2007, 27(1), 89–95.
YANGA, Y., H. MAA., X.CHENA., C.ZHUA., and X. LI. Effect of incineration temperature on chromium speciation in real chromium-rich tannery sludge under air atmosphere. Environmental Research. 2020, 183, 109159.
NORBY, P., A. NORLUND., H. FJELLVAG., and M. NIELSEN. The crystal structure of Cr8O21 determined from powder diffraction data:Thermal transformation and magnetic properties of a chromium-chromate-tetracchromate. Journal of Solid State Chemistry. 1991, 94(2), 281-293.
MAO, L., B. GAO., N. DENG., L. LIU, and H. CUI. Oxidation behavior of Cr(III) during thermal treatment of chromium hydroxide in the presence of alkali and alkaline earth metal chlorides. Chemosphere. 2016, 145,1-9.
LAZAU, R.I., C. PACURAIU., R. BECHERESCU., and R. IANOS. Ceramic pigments with chromium content from leather wastes. Journal of the European Ceramic Society. 2007, 27, 1899–1903.
GRYGAR, T., P. BEZDICKA., J. DEDECEK., E. PETROVSKY., and O. SCHNEEWEISS. Fe2O3-Cr2O3 SYSTEM REVISED. Ceramics – Silikáty. 2003, 47(1), 32-39.
TUYEN, T.N., N.D. QUYEN., and T.B. LAM. Synthesis of FexZn1-xCr2O4 brown ceramic pigment by starch assisted sol-gel process. Hue University Journal of Science: Natural Science. 2019, 128(1B), 13–19.
สำนักงานมาตรฐานผลิตภัณฑ์อุตสาหกรรม. มอก. 32-2546. วิธีทดสอบตะกั่วและแคดเมียมที่ละลายจากภาชนะเซรามิก ภาชนะเซรามิกแก้ว และภาชนะแก้วที่ใช้กับอาหาร. กรุงเทพฯ, ประเทศไทย: สำนักงานมาตรฐานผลิตภัณฑ์อุตสาหกรรม, 2546.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Bulletin of Applied Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.









