Batch Modification for Glass Melting Energy Reduction
DOI:
https://doi.org/10.60136/bas.v1.2012.177Keywords:
Glass, Glass melting, Energy reductionAbstract
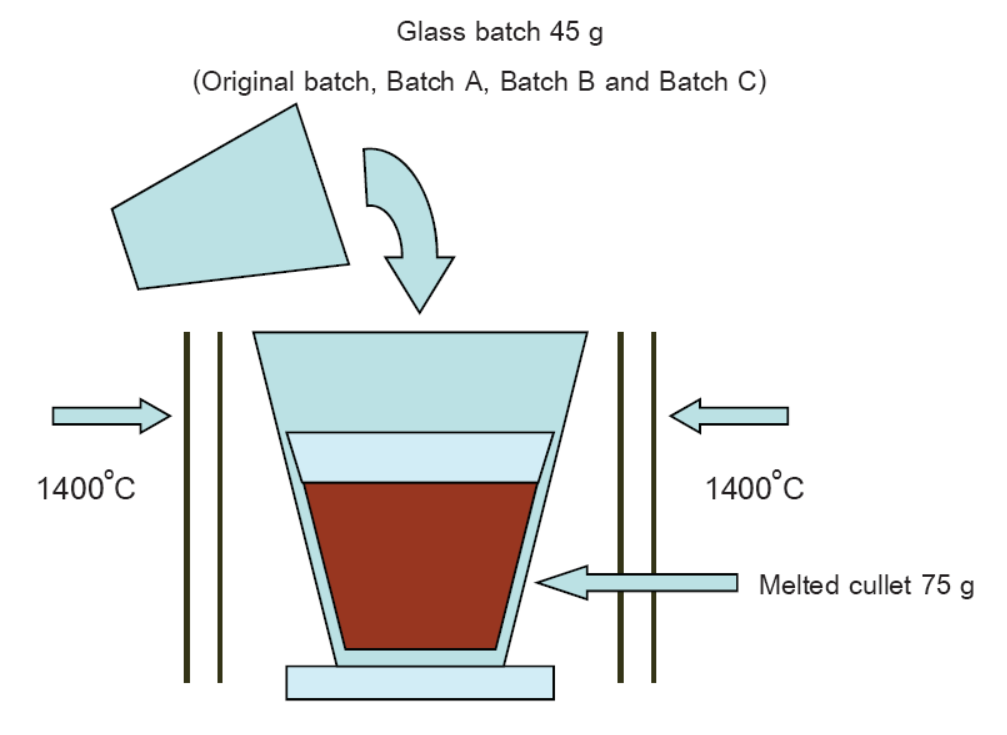
Soda-lime silicate glass is produced from the commercial batch composing of sand (SiO2), soda ash (Na2CO3), dolomite (CaMg(CO3)2) and alumina (AI2O3). The batch modification is done by replacing wollastonite (CaSiO3) with magnesium oxide (MgO), and potassium feldspar (KAISi3O8) or pyrophyllite (Al2Si4O10(OH)2) with alumina. From thermodynamic calculations and Batch-Free Time testing, it was found that the modification batch had a lower melting energy (exploited heat) and higher melting ability, respectively. The modified-batch glasses also had similar chemical compositions and physical properties comparing with the original batch. This implies that the batch modification can reduce energy consumption and is probable to introduce into a large-scale glass production.
References
Beerkens, R. G. and van Limpt, J. A. C. “Evaporation in industrial glass melt furnace,” J. Glass. Sci Technol., 2001, 74(9): 245-257.
Cable, M. “Mechanization of glass manufacture.” J. Am. Ceram. Soc. 1999, 82, 1093–1112.
Madivate, C., Müller, F. and Wilsmann, W. “Thermochemistry of glass melting process energy requirement in melting soda-lime-silica glasses from cullet containing batches.” Glastech. Ber. 1996, 69(6): 167-178.
Carty, W. M., Kim U. and Sinton, C. W. “Selective batching improved commercial glass melting. “Am. Ceram. Soc. Bull. 2004, 83, 28-32.
Montoya, G. B., Torres-Martines, L. M., Quintana, P. and Ibarra, J. “Alternative batch compositions in the glass-forming region of the Na20-CaO-SiO2 system.” J. Non-Cryst. Solids. 2003, 329, 22–26.
Meechoowas, E., Ketboonruang, P., Tapasa, K., and Jitwatcharakomol, T. “Improve melting efficency by Batch-to melt conversion.” Procedia Engineering. 2012, 32, 956-961.
Tapasa, K., and Jitwatcharakomol, T. “Thermodynamic calculation of exploited heat used in glass melting furnace.” Procedia Engineering. 2012, 32, 969-975.
Bieler, B. H. and Bunting, A. J. “Batch-free time vs crucible volume and soda type in glass melting.” Am. Ceram. Soc. Bull. 1984, 11, 1405-1407.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2012 Bulletin of Applied Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.









