Micellar Effect on the Kinetics of Ethanol Oxidation by Potassium Permanganate in Acidic Medium
DOI:
https://doi.org/10.14456/nujst.2023.39Keywords:
Kinetics, Sodium Dodecyl Sulphate (SDS), Oxidation, Potassium Permanganate, EthanolAbstract
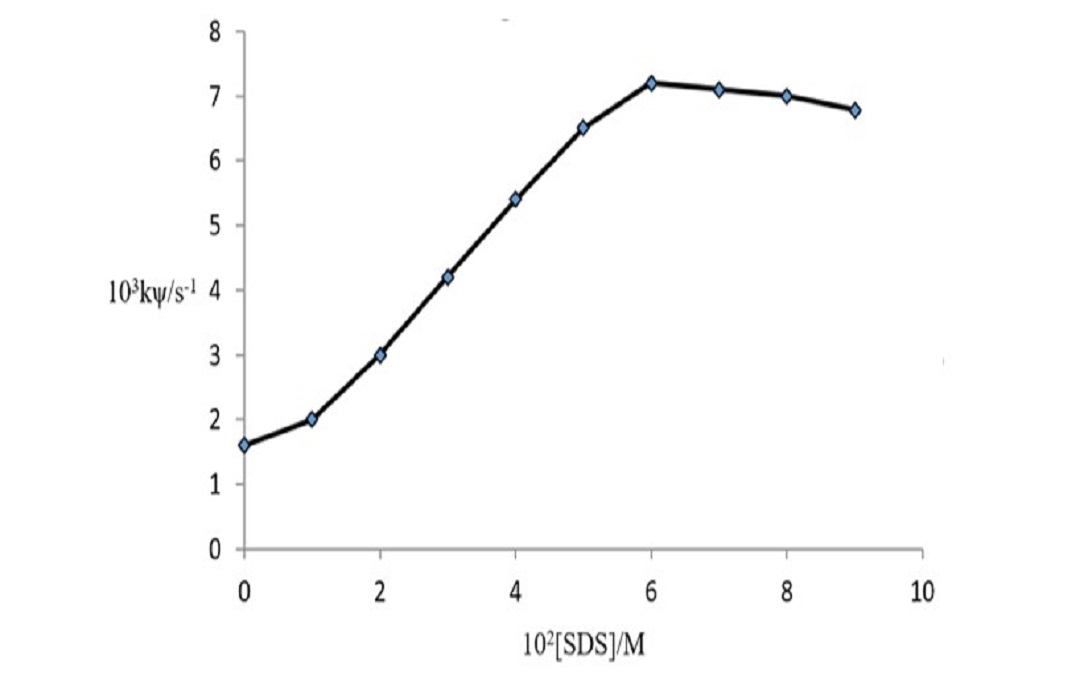
The catalytic effect of sodium dodecyl sulphate (SDS) micelles on the oxidation of alcohols by potassium permanganate in an acidic medium was investigated using a double beam Unicam-1800 Schmadzu UV/Visible- spectrophotometer at λmax 525 nm under pseudo-first-order kinetics. The kinetic study followed a first-order dependence each on [KMnO4], [Ethanol] and fractional order to [H+]. The reaction was catalyzed by SDS at low concentration values and invariant at higher [SDS]. The catalytic effect may be attributed to the solubilization and incorporation of the reactants into the stern layer of the micelles and the inhibition at higher levels of SDS is attributed to the repulsion between the protonated oxidant accumulated on the surface of the micelle and the surfactant counter ions. The results are discussed in terms of the Pseudo-Phase Model as proposed by Menger-Portnoy and Piszkiewicz's positive cooperativity model. The activation parameters were obtained from Erying’s equation as ∆H# 4.16 kJ mol-1, ∆S# -0.198kJK-1 mol-1 and ∆G# 63.16 kJmol-1. The negative ∆S# suggests an ordered transition state. The micellar rate constant, km and the binding constant Ks, were 7.45 x 10-3 s-1 and 40.35 mol dm-3 respectively. The binding constant indicates the existence of surfactant-substrate interaction and the value of n>1 suggests positive cooperativity. The study showed that electrostatic/hydrophobic interactions play a significant role in the micellar-influenced reaction and further affirmed the fact that all chemical reactions involving protonation are catalyzed by anionic surfactants.
References
Agnihotri, M., & Tiwarrii, S. (2021). Oxidation of Unsaturated Alcohols by N-Chlorosaccharin in Micellar Medium. International Journal of Theoretical & Applied Sciences, 13(2), 12-16.
Al-Blewi, F. F., Al-Lohedan, H. A., Rafiquee, M. Z. A., & Issa, Z. A. (2013). Kinetics of Hydrolysis of Procaine in Aqueous and Micellar Media. International Journal of Chemical Kinetics, 45(1), 1-9. http://dx.doi.org/10.1002/kin.20735
Al-Shamay, M. N., Al-Lohedan, H. A., Rafiquee, M. Z. A., & Issa, Z. A. (2012). Micellar Effects on Aromatic Nucleophilic Substitution by the ANRORC Mechanism. Hydrolysis of 2-Chloro-3,5-Dinitropyridine. Journal of Physical Organic Chemistry, 25(8), 713-719. http://dx.doi.org/10.1002/Poc.2908
Astray, G., Cid, A., Manso, J. A., Mejuto, J. C., Moldes, o., & Morales, J. (2011). Influence of Anionic and Nonionic Micelles upon Hydrolysis of 3-hydroxyl- carbofuran. International Journal of Chemical Kinetics, 43, 402-408. http://dx.doi.org/10.1002/kin.20563
Brinchi, L., Germani, R., Goracci, I., Savelli, G., Spreti, N., & Rdiproflia, R. (2006). Temperature Effects upon Aqueous Micellar Assisted Decarboxylation of 6-Nitrobenzisoxazole-3-Carboxylate and its 5-Methyl Derivatives. Journal of Colloid and Interface Science, 298(1), 426-431. http://dx.doi.org/10.1016/j.jcis.2005.11.050
Buton, C. A. (2006). The Dependence of Micellar Rate Effect upon Reaction Mechanism. Advances in Colloid and Interface Science, 2006(123-126), 333-343. http://dx.doi.org/10.1016/j.cis2006.05.008
Dar, M. Y., Andrabi, S. M. Z., & Khan, Z. (2006). Effects of Cationic and Anionic Surfactants on the Addition-Elimination-Type Interaction between o-toluidine and D-glucose. Colloid and Polymer Science, 284, 1008-1015. http://dx.doi.org/10.1007/s00396-005-1450-y
Dar, M. Y., Ilyas, M., Kumar, P., & Khan, Z. (2006). Effects of Anionic and Cationic Micelles on the Oxidation of D-dextrose by Diperiodatoargentate (III). Colloid and Polymer Science, 285, 315-321. http://dx.doi.org/10.1007/s00396-006-1573-9.
Das, A. K., Kar, D., & Mondal, S. K. (2001). Picolinic Acid Assisted Three- Electron Chromic Acid Oxidation of dl-Mandelic Acid in Aqueous Micellar Media: A Kinetic Study. Inorganic Reaction Mechanisms, 3, 83. http://dx.doi.org/10.3184/146867808X339296
Dash, A. C., Dash, B., & Panda, D. (1985). Hydrolysis of Imine. Micellar Effects upon the Spontaneous Acid, Base and Copper (II) Ions Induced Hydrolysis of N-Salicylidene-2-amino thiazole and N-Salicylidene-2-aminopyridine. The Journal of Organic Chemistry, 50, 2905-2910.
Din, K., Hartani, K., & Khan, Z. (2001). Effect of Micelles on the Oxidation of Oxalic Acid by Chromium (VI) in the Presence and Absence of Manganese (II). Colloids and Surfaces A, 193, 1-13. http://dx.doi.org/10.1016/s0927-7757(01000475-7
Ghose, S. K., Basu, A., Saha, R., Ghose, A., Mukherjee, K., & Saha, B. (2012). Micellar Catalysis on Picolinic Acid Promoted Hexavalent Chromium Oxidation of Glycerol. Journal of Coordination Chemistry, 65(7), 1158-1177. http://dx.doi.org/10.1080/00958972.2012.669035
Ghosh, K.K., & Sar, S.K. (1997). Effect of Micelles on the Acid Hydrolysis of N-Phenylbenzohydroxamic Acid. Reaction Kinetics and Catalysis Letters, 61(1), 193-199.
Ghosh, K.K., & Verma, S.K. (2009). Effects of Head Group of Cationic Surfactants on the Hydrolysis of p-Nitrophenyl Acetate Catalyzed by a Chymotrypsin. International Journal of Chemical Kinetics, 41(6), 377-381. http://dx.doi.org/10.1002/kin.20408
Gille, K., Knoll, H., & Quintzsch, K. (1999). Rate Constants of the Thermal cis-trans Isomerization of Azobenzene Dyes in Solvents, Acetone/Water Mixtures and in Micro Heterogeneous Surfactant Solution. International Journal of Chemical Kinetics, 31(5), 337-350. http://dx.doi.org/10.1002/(SICI)1097-4601(1999)31:5<337
Hans-Friedrick, E., & Andeas, D. (1978). Kinetic Study of Naturally Occuring Surfactants. Journal of Colloid and Interface Science, 64(2), 386-388.
Hassan, M., Al-Hakimi, A. N., & Alahmadi, M. D. (2011). Kinetics of Oxidation of Aliphatic Alcohols by Potassium Dichromate in Aqueous and Micellar Media. South African Journal of Chemistry, 64, 237-240.
Hsieh, H. J., Nair, G. R., & Wu, W. T. (2006). Production of Ascoryl Palmitate by Surfactant Coated Lipase in Organic Media. Journal of Agricultural and Food Chemistry, 54(16), 5777-5781. http://dx.doi.org/10.1021/jf060089d
Hunt, I., & Johnson, C. D. (1991). Diels Alder Reaction of Fumaronitrile and Cyclopentadiene in Water: The Influence of Cosolutes. Journal of the Chemical Society, Perkin Transactions. 2(7), 1051-1056. http://dx.doi.org/10.1039/P29910001051
Kabir-ud –Din, D., Morshed, A. M. A., & Khan, Z. (2002). Influence of Sodium Dodecyl Sulfate/ Triton-X Micelles on the Oxidation of D- fructose by Chromic Acid in Presence of HClO4. Carbohydrate Research, 337, 1573-1583.
Latona, D. F., & Akinola, E. A. (2022). Dynamics of CTAB Micelle Mediated Reaction of Fuchsin Degradation in Alkaline Medium. Ovidius Unuiversity Annals of Chemistry, 33(2), 108-112. http://dx.doi.org/10.2478/auoc-2022-0016
Menger, F. M., & Portnoy, C. E. (1967). Chemistry of Reactions Proceeding inside Molecular Aggregates. Journal of the American Chemical Society, 89, 4698. http://dx.doi.org/10.4236/oalib.1100413
Mondal, S. K., Das, M., Kar, d., & Das, A. K. (2001). Micellar Effect on the Reaction of Chromium (VI) Oxidation of Formaldehyde in the Presence and Absence of Picolinic Acid in Aqueous Acid Media: A Kinetic Study. Indian Journal of Chemistry, 40A, 352-360.
Moore, B. M., & Flurkey, W. H. (1990). Sodium Dodecyl Sulphate Activation of a Plant Polyphenoloxidase. Effect of Sodium Dodecyl Sulphate on Enzymatic and Physical Characteristics of Purified Broad Bean Polyphenol Oxidase. Journal of Biological Chemistry, 265(9), 4982-4988. http://dx.doi.org/10.1016/s0021-9258(19)34072-4
Mubofu, E. B., & Engberts, J. B. E. N. (2007). Surfactant Assisted Specific-Acid Catalysis of Diels-Alder Reaction in Aqueous Media. Journal of Physical Organic Chemistry, 20(10), 764-770. http://dx.doi.org/10.1002/Poc.1240
Pandey, S., & Upadhyay, S. K. (2005). Effect of Cationic Micellar Aggregates on the Kinetics of Oxidation of Aminoalcohols by N-Bromosuccinimide in Alkaline Medium. Journal of Colloid and Interfacescience, 285, 789-794. http://dx.doi.org/10.1016/j.jcis.2004.01.085
Park, K. M., Kwom, C. W., & Choi, S. J. (2013). Thermal Deactivation Kinetics of Pseudomonas Fluorescence. Lipase Entrapped in AOT/Isooctane Reverse Micelles. Journal of Agricultural and Food Chemistry, 61(39), 9421-9427. http://dx.doi.org/10.1021/jf402539m
Perez-Benito, E., & Rodenas, E. (1991). Influence of Sodium Dodecyl Sulfate Micelles on the Oxidation of Alcohols by Chromic Acid. Langmuir, 7, 2, 232-237. http://dx.doi.org/abs/10.1021/la00050a005
Piszkiewicz, D. (1976). Micelle Catalysed Reactions are Models of Enzyme Catalysed Reactions Which shoe Positive Homotropic Interactions. Journal of the American Chemical Society, 98(10), 3053-3055. http://dx.doi.org/10.1021/ja00426a083
Rodriguez, A., delMarGraciani, M., Munoz, M., & Moya, M. I. (2000). Study of the Bromide Oxidation of Bromate in Zwitterionic Micellar Solutions. International Journal of Chemical Kinetics, 32(6), 388-394. http://dx.doi.org/10.10021/(SICI)1097-4601(2000)32:6<388
Saha, B., Sarka, S., & Chowdbury, K. M. (2008). Micellar Effect on Quinquivalent Vanadium Ion Oxidation of D-Glucose in Aqueous Acid Media: A Kinetic Study. International Journal of Chemical Kinetics, 40(5), 282-286. http://dx.doi.org/10.1002/kin.20314
Samiey, B., & Toosi, A. R. (2009). Kinetics Study of Malachite Green Fading in the Presence of TX-100, DTAB and SDS. Bulletin of the Korean Chemical Society, 30(9), 2051-2056. http://dx.doi.org/10.5012/bkcs.2009.30.9.205
Shi, Z., Sigma, M. E., Ghosh, M. M., & Dabestani, R. (1997). Phytolysis of 2-Chlorophenol Dissolved in Surfactant Solution. Environmental Science Technology, 31(12), 3581-3587. http://dx.doi.org/10.1021/es9703279
Tascioglu, S. (1996). Micellar Solutions as Reaction Media. Tetrahedron, 52, 11113. http://dx.doi.org/10.1016/0040-4020(96)00669-2
Vijapure, Y. A. (2017). Kinetics and Mechanism of Micellar Catalyzed Oxidation Reactions of Aliphatic Acid Hydrazides to their Correseponding Acids by Vanadium (V). Journal of Emerging Technologies and Innovation Research, 4(12), 7-14.
William, R. J., Phillips, J. W., & Mysel, K. J. (1985). Critical Micelle Concentrations of Sodium Dodecyl Sulphate at 25oC. Transactions of the Faraday Society, 51, 728-737. http://dx.doi.org/10.1039/TF955100728
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Naresuan University Journal: Science and Technology (NUJST)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.













