Prevalence and Risk Factors of Cardiotoxicity in Patients with Multidrug Resistant Tuberculosis Infection Receiving a Shorter All-oral Bedaquiline-containing Regimen
DOI:
https://doi.org/10.14456/nujst.2023.32Keywords:
QT prolongation, bedaquiline, hypokalemia, multidrug-resistant tuberculosis, risk factorAbstract
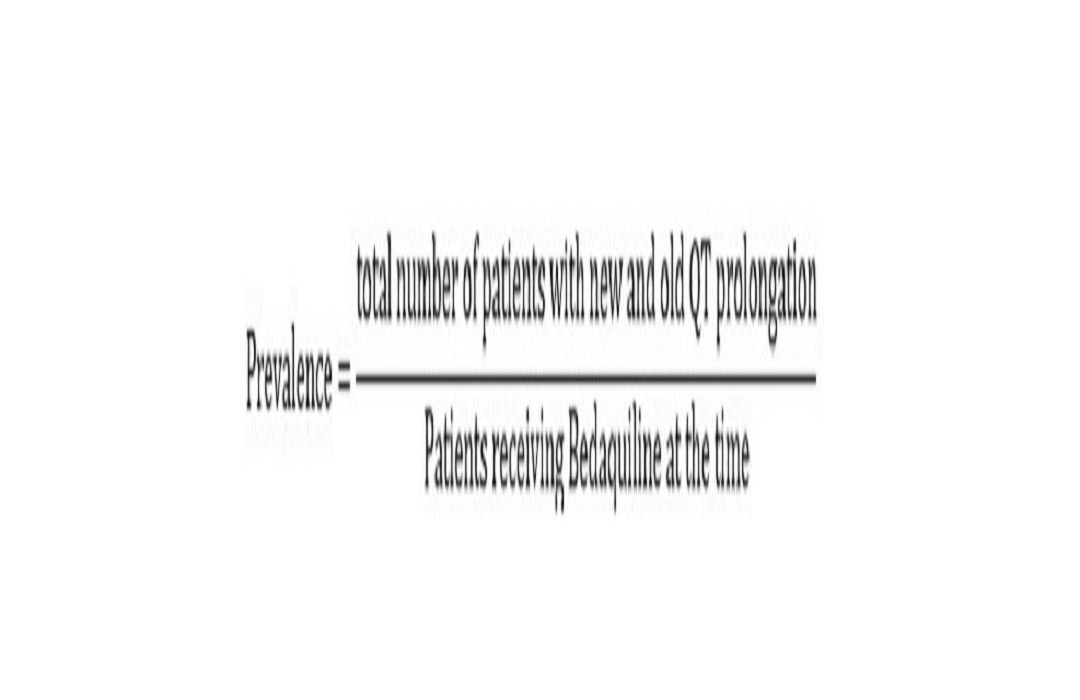
The most important adverse effect of bedaquiline is QT prolongation. There have not been any definitive studies on the prevalence of QT prolongation caused by bedaquiline drugs used in Thailand. Therefore, the purpose of this retrospective chart review study was to estimate the prevalence and risk factors of QT prolongation in patients with multidrug-resistant tuberculosis (MDR-TB) infection who were receiving a shorter all-oral bedaquiline-containing regimen. The data of the MDR-TB patients who received this treatment regimen between June 1, 2020, and December 31, 2021, at the Central Chest Institute of Thailand and Makaruk Hospital were collected. The event of QTc prolongation and risk factors, including QTc baseline, gender, QT prolongation diagnosed by physician, potassium level, comorbidities, and other drugs used in the shorter all-oral Bedaquiline-containing regimen, were recorded. Results showed that, from 33 patients (19 men and 14 women, age 43.24±15.79 years) with multidrug-resistant tuberculosis infection who received treated under this regimen, 27 (81.82%) of the patients received levofloxacin-based regimen, and 2 of the patients (6.06%) had QT prolongation. Other factors that may contribute to the development of QT prolongation are hypokalemia and other drugs used in this regimen. This study may lead to the development of a risk assessment tool for monitoring QT prolongation in patients who receive the shorter all-oral bedaquiline-containing regimen.
References
Abdelwahab, M. T., Court, R., Everitt, D., Diacon, A. H., Dawson, R., Svensson, E. M., … Denti, P. (2021). Effect of clofazimine concentration on QT prolongation in patients treated for tuberculosis. Antimicrobial Agents and Chemotherapy, 65(7), e0268720. https://doi.org/10.1128/aac.02687-20.
Borisov, S., Filippov, A., Ivanushkina, T., Ivanova, D., & Litvinova, N. (2016). Bedaquiline-containing regimens for MDR TB treatment – focus on the safety. European Respiratory Journal, 48(60), OA3518. https://doi.org/10.1183/13993003.congress-2016.oa3518
Borisov, S. E., Dheda, K., Enwerem, M., Romero Leyet, R., D'Ambrosio, L., Centis, R., … Battista, G. (2017). Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: A Multicentre study. European Respiratory Journal, 49(5), 1700387. https://doi.org/10.1183/13993003.00387-2017
Boutjdir, M., Lazzerini, P., Capecchi, P., Laghi-Pasini, F., & El-Sherif, N. (1970). Potassium channel block and novel autoimmune-associated long qt syndrome. Cardiac electrophysiology clinics, 8(2), 373-384. Retrieved from https://www.semanticscholar.org/paper/Potassium-Channel-Block-and-Novel-Long-QT-Syndrome.-Boutjdir-Lazzerini/eacdc1783e88858a46cceb849f0b9acc715e82cf
Briasoulis, A., Agarwal, V., & Pierce, W. J. (2011). QT prolongation and Torsade de Pointes induced by fluoroquinolones: Infrequent side effects from commonly used medications. Cardiology, 120(2), 103–110. https://doi.org/10.1159/000334441
Deoghare, S. (2013). Bedaquiline: A new drug approved for treatment of multidrug-resistant tuberculosis. Indian Journal of Pharmacology, 45(5), 536. https://doi.org/10.4103/0253-7613.117765
Department of disease control, division of tuberculosis. (2020). Guidelines for choosing a drug regimen to treat drug-resistant tuberculosis. Retrieved from https://tobe.pyomoph.go.th/backoffice/files/40664.pdf
Nachimuthu, S., Assar, M. D., & Schussler, J. M. (2012). Drug-induced QT interval prolongation: Mechanisms and clinical management. Therapeutic Advances in Drug Safety, 3(5), 241–253. https://doi.org/10.1177/2042098612454283
Noori, M., Nejadghaderi, S. A., Sullman, M. J., Carson‐Chahhoud, K., Kolahi, A. A., & Safiri, S. (2022). Epidemiology, prognosis and management of potassium disorders in Covid‐19. Reviews in medical virology, 32(1), e2262.
Patel, H., Pawara, R., Pawara, K., Ahmed, F., Shirkhedkar, A., & Surana, S. (2019). A structural insight of bedaquiline for the cardiotoxicity and hepatotoxicity. Tuberculosis, 117, 79–84. https://doi.org/10.1016/j.tube.2019.06.005
Ponte, M. L., Keller, G. A., & Girolamo, G. D. (2010). Mechanisms of drug induced QT interval prolongation. Current Drug Safety, 5(1), 44–53. https://doi.org/10.2174/157488610789869247
Pym, A. S., Diacon, A. H., Tang, S.-J., Conradie, F., Danilovits, M., Chuchottaworn, C., … Dannemann, B. (2015). Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. European Respiratory Journal, 47(2), 564–574. https://doi.org/10.1183/13993003.00724-2015
Roden, D. M. (2004). Drug-induced prolongation of the QT interval. New England Journal of Medicine, 350(25), 2618–2621. https://doi.org/10.1056/nejm200406173502517
Widimsky, P. (2008). Hypokalemia and the heart. European Society of Cardiology, Retrieved from https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-7/Hypokalemia-and-the-heart
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Naresuan University Journal: Science and Technology (NUJST)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.













